Pharmaceutical document attestation or obtaining a Certificate of Pharmaceutical Product (CPP) and Certificate of Origin (CoO) in the UAE is mandatory for pharmaceutical companies aiming to meet regulatory compliance and facilitate exports. Commercial documents attestation is a vital process to authenticate your documents for legal and regulatory purposes. The UAE has stringent regulatory requirements for pharmaceutical products to ensure safety, quality, and compliance. Document attestation and Certificate of Pharmaceutical Product (CPP) issuance are critical for pharmaceutical companies aiming to operate within the UAE or export from it.
Pharmaceutical commercial document attestation is the process of verifying and validating official business papers related to pharmaceuticals for use in the United Arab Emirates. This attestation confirms that your documents are genuine and comply with UAE regulations. Common pharmaceutical documents requiring attestation include: Good Manufacturing Practices (GMP) Certificates Product Registration Certificates Commercial Invoices Certificate of Analysis (COA) Purchase Orders and Contracts ISO Certifications
The documents must first be verified by the relevant issuing authority in the home country. For instance:
Once verified by the issuing authority, documents are submitted to the MOFA for national-level attestation.
The UAE embassy in the home country verifies the documents to ensure they align with UAE regulatory standards.
Upon arrival in the UAE, the documents must be attested by the MOFA for final validation.
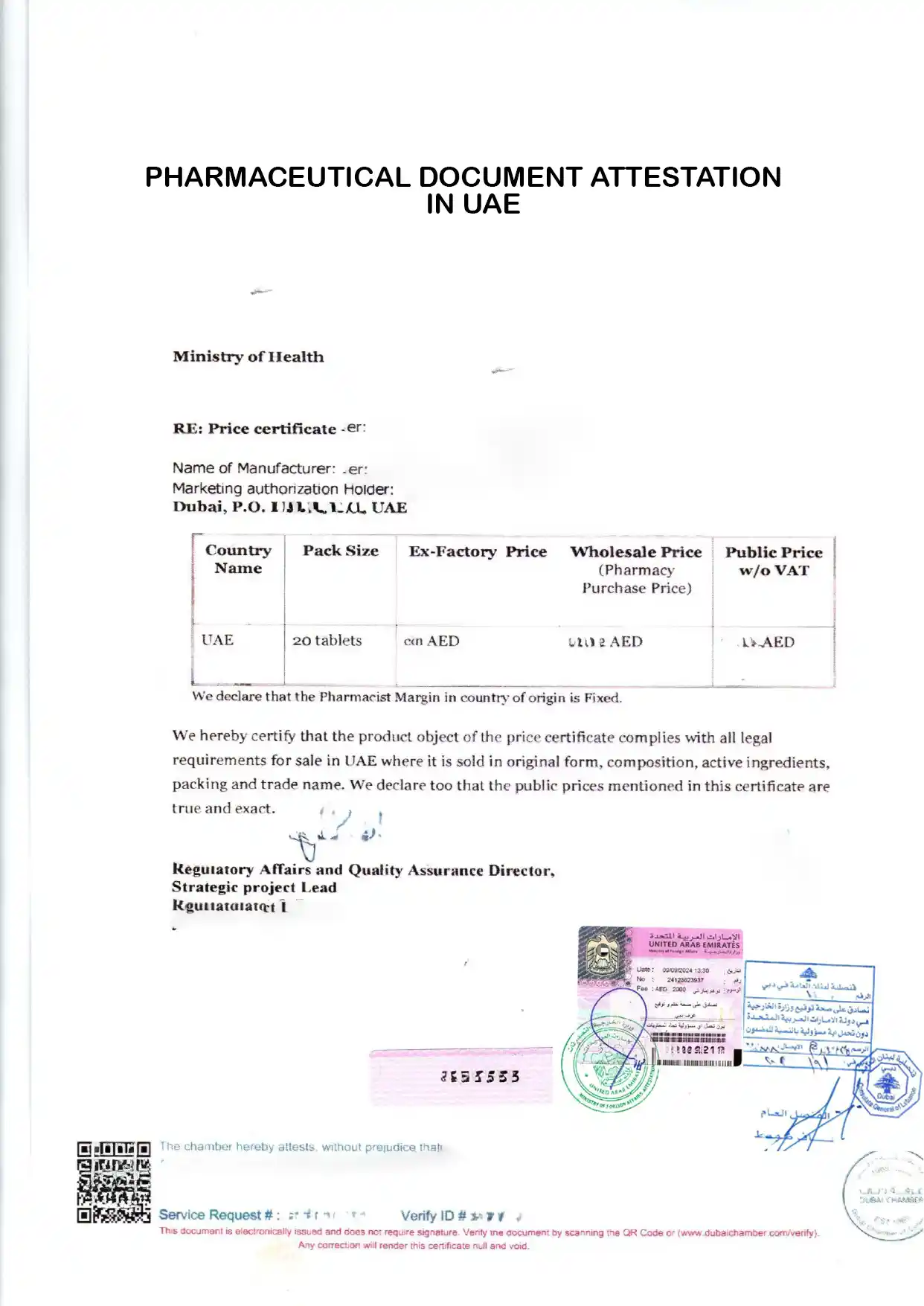
The Certificate of Pharmaceutical Product (CPP) is an essential document issued by the UAE’s Ministry of Health and Prevention (MOHAP) to support pharmaceutical manufacturers in exporting their products. This certification confirms that the product meets international quality standards, making it easier for UAE-manufactured pharmaceuticals to gain regulatory acceptance in global markets. Required Documents To ensure a successful application, prepare the following documents: Product Composition Certificate: Signed and stamped by the authorized person. Details all active and inactive ingredients. Product Insert Leaflet: Approved by MOHAP, signed, and stamped. Manufacturing Details: Confirm compliance with GMP standards.

To obtain a Certificate of Pharmaceutical Product (CPP) for export in the UAE, certain conditions and requirements must be met. The CPP is issued for locally manufactured pharmaceutical products and remains valid for one year from the issuance date, provided the product registration does not exceed its five-year validity period. This ensures the product’s compliance with UAE health regulations and international standards.
Exporting pharmaceutical products across international borders is a complex process that demands meticulous preparation and strict adherence to regulatory standards. Ensuring you have the correct documentation not only facilitates smooth customs clearance but also guarantees compliance with global pharmaceutical regulations. From Certificates of Pharmaceutical Products (CPP) to manufacturing licenses, understanding these essential documents can streamline your export process and ensure market access in key regions.
A Certificate of Pharmaceutical Product (CPP) is a globally recognized document issued in line with the World Health Organization’s (WHO) guidelines. It verifies the quality, safety, and efficacy of pharmaceutical products and confirms compliance with the regulations of the manufacturing country. This document is essential for gaining regulatory approval in import markets and streamlining global trade.
Good Manufacturing Practice (GMP) certification assures that pharmaceutical products meet quality standards throughout the production process. GMP ensures compliance in areas such as manufacturing facilities, employee training, and raw material handling, making it a cornerstone document for international trade.
Standard shipping documents like the Commercial Invoice, Packing List, and Bill of Lading are indispensable for the legal transportation of goods. These documents facilitate smooth customs clearance by detailing product specifications, destination, and transportation methods.
A Free Sale Certificate confirms that a product is legally sold and marketed in its country of origin. Many importing countries require this certificate to verify that the product complies with all local regulations and quality standards.
These licenses authorize manufacturers to produce specific pharmaceutical products. They ensure that manufacturing processes meet international quality and safety standards, making them critical for exporting pharmaceutical goods.
This document certifies the country of manufacture for a product. It is crucial for customs clearance and determining eligibility for trade agreements or tariff reductions.
For exporters dealing in veterinary pharmaceuticals, the Veterinary Medicines Directorate Certificate ensures compliance with safety and quality standards, particularly in markets like the UK.
For companies exporting to India, a Bureau of Indian Standards (BIS) certification demonstrates that products meet local safety and quality requirements.
Issued by Ireland’s Health Products Regulatory Authority, these certificates confirm compliance with the country’s safety and efficacy standards. They are essential for companies exporting to or from the Irish market.
The European Medicines Agency (EMA) issues comprehensive reports on pharmaceutical products marketed within the European Union. These reports ensure regulatory compliance and facilitate seamless market entry across EU member states.
The Certificate of Origin (CoO) is a crucial document for exporters and producers in the UAE. It certifies that goods exported are manufactured, produced, or processed in the UAE, ensuring compliance with international trade regulations. This guide simplifies the process for obtaining a CoO, covering the steps, fees, requirements, and more.
A Certificate of Origin validates the origin of goods being exported and is essential for preferential and non-preferential trade agreements. It is issued by the Ministry of Economy (MoEc) and facilitates smooth customs clearance, regulatory compliance, and access to trade benefits in destination countries.
To issue a Certificate of Origin, applicants must meet the following conditions:
Visit the MoEc portal and select “Certificate of Origin” from the list of e-Services.
Log in using your UAE PASS credentials for secure access to the system.
Choose the organization for which the CoO will be issued:
Provide all necessary details, including invoice and consignee information. Attach the required documents.
Complete the payment via the portal. You can pay immediately or choose the “Pay Later” option.
The MoEc will review the submitted application. If any information or documentation is missing, you will receive a notification to correct it.
Once approved, you can download the CoO from the workspace under the “Outputs” section.
The type of documentation depends on the production category:
At GloboPrime Corporate Services, we specialize in providing end-to-end services for commercial document attestation. Our expertise ensures compliance, accuracy, and timely completion of the process.
What We Offer:
Embassies Attestation in UAE We can assist you with: